Synthetic Biology
We are employing engineering principles to model, design and build synthetic gene circuits and programmable cells, in order to create novel classes of diagnostics & therapeutics. We are also using deep learning approaches to discover new genetic parts and enhance the synthetic biology design process.
Antibiotics & AI
As part of the Antibiotics-AI Project, we are harnessing the power of artificial intelligence (AI) to discover novel classes of antibiotics and rapidly understand how they work. We are also using deep learning approaches for the de novo design of new antibiotics and the development of combination treatments.
The Collins Lab is part of the Institute for Medical Engineering and Science (IMES) and the Department of Biological Engineering at MIT, the Harvard-MIT Program in Health Sciences and Technology (HST), the Broad Institute of MIT and Harvard, and the Wyss Institute for Biologically Inspired Engineering at Harvard. At MIT, our lab is part of the Synthetic Biology Center, the Computational and Systems Biology Initiative, and the Microbiology Graduate Program.
RECENT PUBLICATIONS
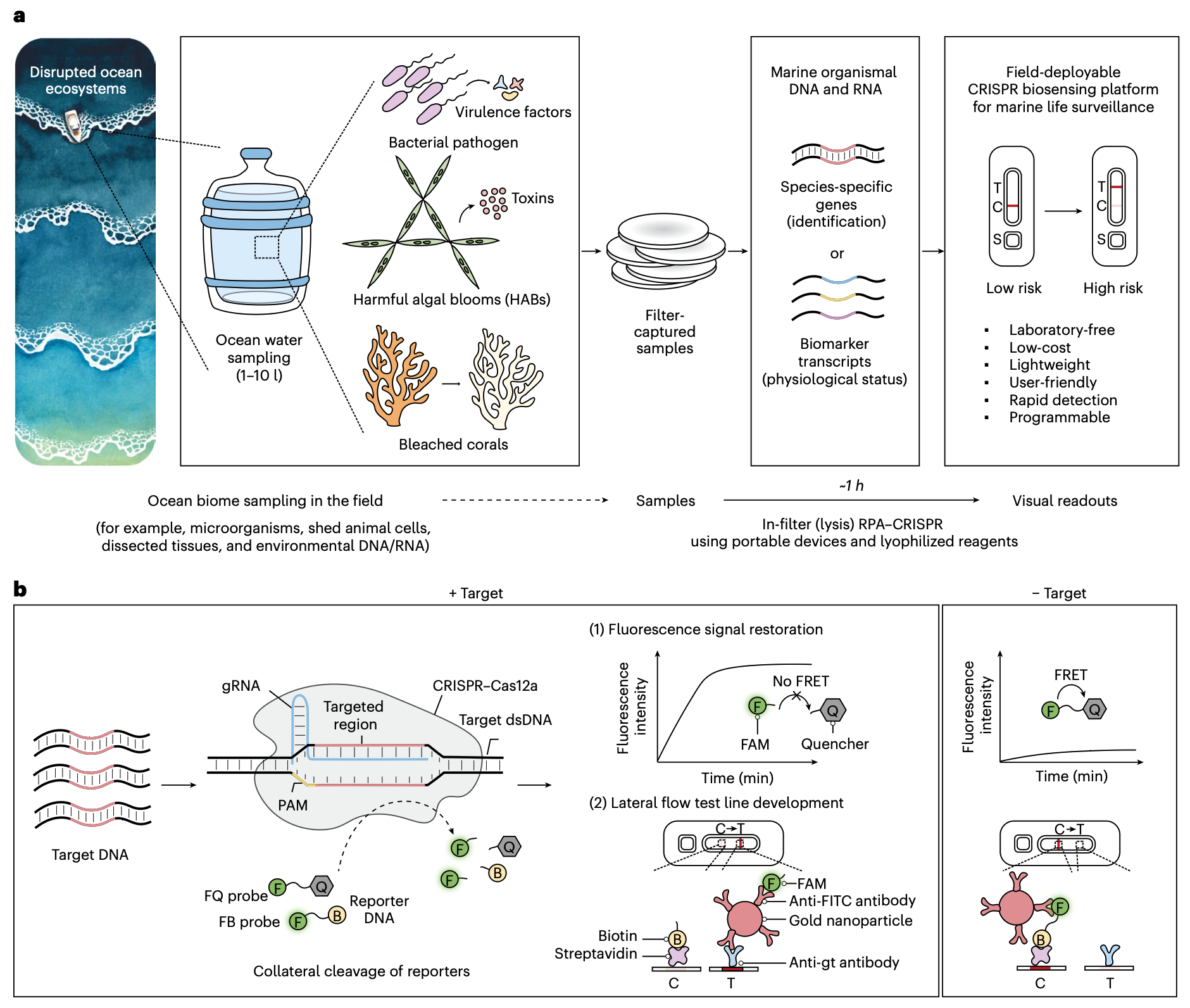
A field‑deployable CRISPR-based biosensing platform for monitoring marine ecosystems
Nayoung Kim, Daniel S. Collins, Nina M. Donghia, Benjamin S. Miller, Hani M. Sallum, Silvi R. Lybbert, Elena Perini, James B. Niemi, James J. Collins and Peter Q. Nguyen
Ocean ecosystems are undergoing accelerating disruption from human impacts such as climate change. Warming ocean temperatures drive pathogenic outbreaks, increase harmful algal blooms and cause coral stress. These can have serious consequences for marine ecosystems, human health and the aquaculture industry, representing a critical One Health issue. Monitoring key marine species offers valuable insights, but current methods are resource-intensive, low-resolution and unsuitable for frequent deployment. Here we introduce a low-cost, field-deployable CRISPR biosensing platform for detecting marine organismal DNA and RNA. Harnessing the programmability of CRISPR diagnostics for environmental biosurveillance, we demonstrate versatility across three climate-linked indicators: Vibrio spp., Pseudo-nitzschia spp. and heat-stressed corals. Portable 3D-printed processor and incubator devices enable direct processing of filter-captured samples with temperature control. Field readiness is reinforced by lyophilized reagents, lateral flow readouts, dropper-based handling and a two-step multiplexed workflow, delivering results within 1 hour without laboratory instruments. Benchmarking with authentic pathogens and environmental seawater confirmed seawater tolerance and robust detection of 10⁸ colony-forming units per filter of Vibrio pathogens, equivalent to 10² copies per microlitre for 1 litre of filtered sample. This decentralized platform reduces barriers to routine monitoring and can provide early warnings of ecosystem disturbances, while supporting One Health initiatives in the marine space.
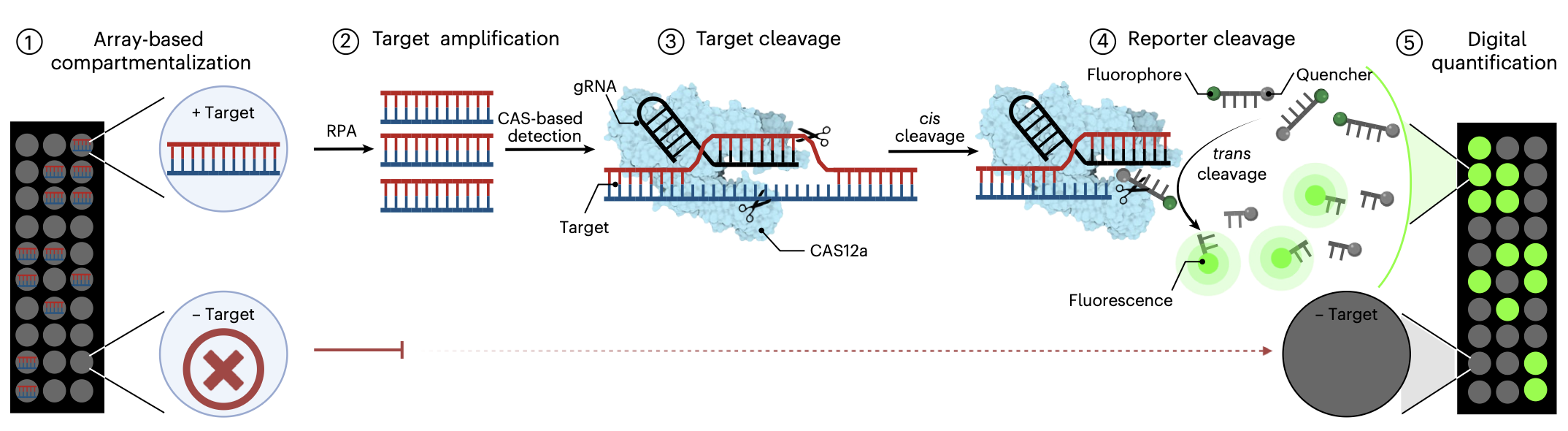
Digital CRISPR-based diagnostics for quantification of Candida auris and resistance mutations
Justin C. Rolando, Anton Thieme, Nicole E. Weckman, Nayoung Kim, Helena de Puig, Xiao Tan, Emily Cotnoir, Vishnu Chaturvedi, James J. Collins and David R. Walt
Nature Biomedical Engineering (2026)
Candida auris, an increasingly prevalent fungal pathogen, requires both rapid identification and antifungal susceptibility testing to enable proper treatment. This study introduces digital SHERLOCK (dSHERLOCK), a platform that combines CRISPR/Cas nucleic acid detection, single-template quantification and real-time kinetics monitoring. Assays implemented on this platform display excellent sensitivity to C. auris from major clades 1–4, while maintaining specificity when challenged with common environmental and pathogenic fungi. dSHERLOCK detects C. auris within 20 min in minimally processed swab samples and achieves sensitive quantification (1 c.f.u. µl⁻¹) within 40 min. To address antifungal susceptibility testing, we develop assays that detect mutations that are commonly associated with azole and echinocandin multidrug resistance. We use machine learning and real-time monitoring of reaction kinetics to achieve highly accurate simultaneous quantification of mutant and wild-type FKS1 SNP alleles in fungal populations with mixed antifungal susceptibility, which would be misdiagnosed as completely susceptible or resistant under standard reaction conditions. Our platform’s use of commercially available materials and common laboratory equipment makes C. auris diagnostics widely deployable in global healthcare settings.
Active learning framework leveraging transcriptomics identifies modulators of disease phenotypes
Benjamin DeMeo, Charlotte Nesbitt, Samuel A. Miller, Daniel B. Burkhardt, Inna Lipchina, Doris Fu, Peter Holderreith, David Kim, Sergey Kolchenko, Artur Szalata, Ishan Gupta, Christine Kerr, Thomas Pfefer, Raziel Rojas-Rodriguez, Sunil Kuppassani, Laurens Kruidenier, Parul B. Doshi, Mahdi Zamanighomi, James J. Collins, Alex K. Shalek, Fabian J. Theis, Mauricio Cortes
Science (2025)
Phenotypic drug screening remains constrained by the vastness of chemical space and technical challenges scaling experimental workflows. To overcome these barriers, computational methods have been developed to prioritize compounds, but they rely on either single-task models lacking generalizability or heuristic-based genomic proxies that resist optimization. We designed an active deep-learning framework that leverages omics to enable scalable, optimizable identification of compounds that induce complex phenotypes. Our generalizable algorithm outperformed state-of-the-art models on classical recall, translating to a 13-17x increase in phenotypic hit-rate across two hematological discovery campaigns. Combining this algorithm with a lab-in-the-loop signature refinement step, we achieved an additional two-fold increase in hit-rate and molecular insights. In sum, our framework enables efficient phenotypic hit identification campaigns, with broad potential to accelerate drug discovery.
Convergence of nanotechnology and CRISPR-based diagnostics
Midori Johnston, Schan Dissanayake-Perera, James J. Collins, Molly M. Stevens, and Can Dincer
Nature Nanotechnology (2025)
In addition to its broad application in genome engineering and therapeutics, clustered regularly interspaced short palindromic repeats (CRISPR) technology provides field-deployable methods for the highly sensitive and selective detection of nucleic acids. From a diagnostic perspective, CRISPR-based assays hold clear clinical potential for identifying a range of both infectious and non-communicable diseases. In this Perspective we evaluate recent nanotechnologies and nanomaterials that have been engineered to interface with CRISPR systems on a nanoscale level to realize the full potential of this versatile diagnostic tool. We assess biomolecules such as enzymes and oligonucleotides, some of the more commonly used synthetic nanoparticles and detection platforms that integrate nanotechnologies in new and innovative ways. We discuss current trends and look ahead to future challenges and opportunities, including non-nucleic acid target detection, pre-amplification-free detection of nucleic acids, the development of wearable devices and integration with artificial intelligence workflows.
A generative deep learning approach to de novo antibiotic design
Aarti Krishnan, Melis N. Anahtar, Jacqueline A. Valeri, Wengong Jin, Nina M. Donghia, Leif Sieben, Andreas Luttens, Yu Zhang, Seyed Majed Modaresi, Andrew Hennes, Jenna Fromer, Parijat Bandyopadhyay, Jonathan C. Chen, Danyal Rehman, Ronak Desai, Paige Edwards, Ryan S. Lach, Marie-Stephanie Aschtgen, Margaux Gaborieau, Massimiliano Gaetani, Samantha G. Palace, Satotaka Omori, Lutete Khonde, Yurii S. Moroz, Bruce Blough, Chunyang Jin, Edmund Loh, Yonatan H. Grad, Amir Ata Saei, Connor W. Coley, Felix Wong, and James J. Collins
Cell (2025)
The antimicrobial resistance crisis necessitates structurally distinct antibiotics. While deep learning approaches can identify antibacterial compounds from existing libraries, structural novelty remains limited. Here, we developed a generative artificial intelligence framework for designing de novo antibiotics through two approaches: a fragment-based method to comprehensively screen >10^7 chemical fragments in silico against Neisseria gonorrhoeae or Staphylococcus aureus, subsequently expanding promising fragments, and an unconstrained de novo compound generation, each using genetic algorithms and variational autoencoders. Of 24 synthesized compounds, seven demonstrated selective antibacterial activity. Two lead compounds exhibited bactericidal efficacy against multidrug-resistant isolates with distinct mechanisms of action and reduced bacterial burden in vivo in mouse models of N. gonorrhoeae vaginal infection and methicillin-resistant S. aureus skin infection. We further validated structural analogs for both compound classes as antibacterial. Our approach enables the generative deep-learning-guided design of de novo antibiotics, providing a platform for mapping uncharted regions of chemical space.